Photons are emitted when an electron in some atom moves from a higher energy state to a lower energy state. The photon has a wavelength which is exactly equal to the energy difference between the two states. The energy of a photon is equal to Planck's constant ( h) times its frequency (f):
Since frequency x wavelength (w) = speed of light (c) for any photon then we have
Thus the energy of a photon is inversely proportional to its wavelength. Short wavelength photons have more energy than long wavelength photons.
The atom below is an idealized case. Its principal energy levels are indicated in units of Electron Volts. As a first step in this procedure do the following:
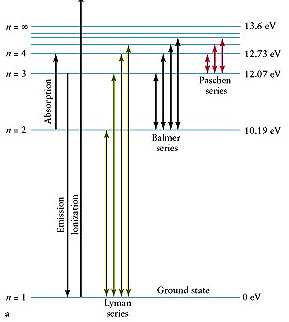
Now we move from the idealized atom to a real hydrogen atom (as shown below). Notice the large separation between the ground state and the first excited state in this atom. Also notice that the seperation between the energy levels gets closer together as you approach the ionization energy (13.6 eV). What this means is that the energy difference between the 3rd and second excited state is smaller than the energy difference between the second and first excited state.
The Balmer series of emission lines from a Hydrogen atom occur when photons make a direct transition from a higher energy level to the first excited state

Determine the wavelengths of the principal Balmer lines, using the Hydrogen Atom applet below. Note to simplify your measurements the "ground state" in this case corresponds to the first excited level and its transitions to this level that define the Balmer series.
So click on any level above the first excited state and then click on the first existed state to make the transition and then observe the emitted wavelengths.
Which transaction produces a blue photon?
You should find that those wavelengths pretty closely match the answer .